shelf life calculator medical device
Describe the organizational processes for determining the phases of testing. All devices are not created equal.
Accelerated Aging Calculator Shelf Life Calculator Packaging Compliance Labs
If Q10 2 and the accelerated aging condition is 55C or.
. Download the Final Guidance Document. Shelf Life of Medical Devices April 1991. Therefore the FDA requires medical device manufacturers to determine a products shelf life before sending the product to market in the United States.
They may claim to perform the same but a device is only as good as its battery life. The elevated temperature condition typically used at Pacific Biolabs for the accelerated aging of medical device packages is 55C. The shelf life of a pharmaceutical product is the maximum time at which the true mean response of a stability limiting characteristic crosses th e acceptance criterion basis for the current.
Accelerated shelf-life testing is commonly used in the medical device industry to accelerate the effects of time on a sterile barrier packaging system to establish shelf life parameters. Accelerated aging parameters with results supported. Test Temperature is typically between 50 to 60C most commonly 55C.
Type the target shelf life Days 2. Shelf life explains duration of the medical device to be stabile to retain the sterility of the package and the device performance. The shelf life of medical devices is determined by putting a device through a variety of testing procedures and many engineers follow this step by procedure to avoid any errors.
The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. Accelerated Aging Time Calculator. The time of simulated aging depends on the temperature at which the products are held.
For example at 55C using an ambient temperature of 25C 65 weeks is equivalent to 1 year on. See how far youand your patientscan go with a Boston Scientific. Type desired TAA TRT Values.
Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product. Accelerated-aging tests are employed to generate this data for. This testing can identify film delineation and leaks.
It ends when the package is open or the shelf life claimed on the. Materials and packaging impacts the shelf life of the device. Ambient storage temperature is typically between 22C to 25C.
Eurofins Medical Device Testings shelf life and accelerated. The medical device industry has long been interested in techniques for predicting the shelf life of polymer-based devices. Accelerated aging testing is an FDA requirement for medical biomedical and pharmaceutical products.
Every medical device is required to be labeled with an expiration date that is supported by shelf-life data. However sometimes it may take many months or even years before you can witness the toll aging takes on a product in real-time This is where accelerated aging also known as accelerated shelf. These accelerated tests help pinpoint possible seal and burst.
22C results in the shortest. Accelerated Aging is a process of putting packaged products into a chamber elevating the test temperature to claim a specific expiration date for a medical device product or package. Q10 value can be changed however default is Q1020 we recommend holding this.
Any medical device brought to market must have an expiration date backed by shelf life testing. A plan for the storage of shelf life samples including storage conditions. Clark GS Shelf Life of Medical Devices FDA DSMA report April 1991.
In the case of a sterile device recognizing shelf life is easy since the packaging has a pull date. Identifying Shelf Life for Non-Sterile Devices. Donohue J and Apostolou S Shelf-Life Prediction for Radiation-Sterilized Plastic Devices Med Dev Diag.
This can also be used to determine the. Manufacturers must account for internal. Put together a sampling plan that includes the amount of devices used as well as how often.
In order to calculate expiry date you should look at Production Date on your. This online service helps you to know how long your product is in good condition. Determining a medical devices shelf life can be one of the more challenging aspects of a new device development program.
It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. This year that interest will increase substantially.

Black Friday Scientific Calculator Calculator Solar Calculator

Playkidz Interactive Toy Cash Register For Kids Sounds Early Learning Play Includes Play Money Handheld Real Scanner Working Scale Calculator Live Microp Toy Cash Register Toy Money Interactive Toys

End Of Service Calculator In Saudi Arabia 2021 Internship Calculator Siemens

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube

Missouri Ems Ambulance Inspection Checklist Ems Ambulance Emergency Medical Inspection Checklist

Studying For Third Year Nbme Shelf Exams Med School Help School Loans Medical School

Panadol Dosage Calculator Iphone App Iphone Apps Iphone App Design Iphone

Multi Dose Calendar Expiration Date Free Calendar Template

Multi Dose Calendar Expiration Date Free Calendar Template
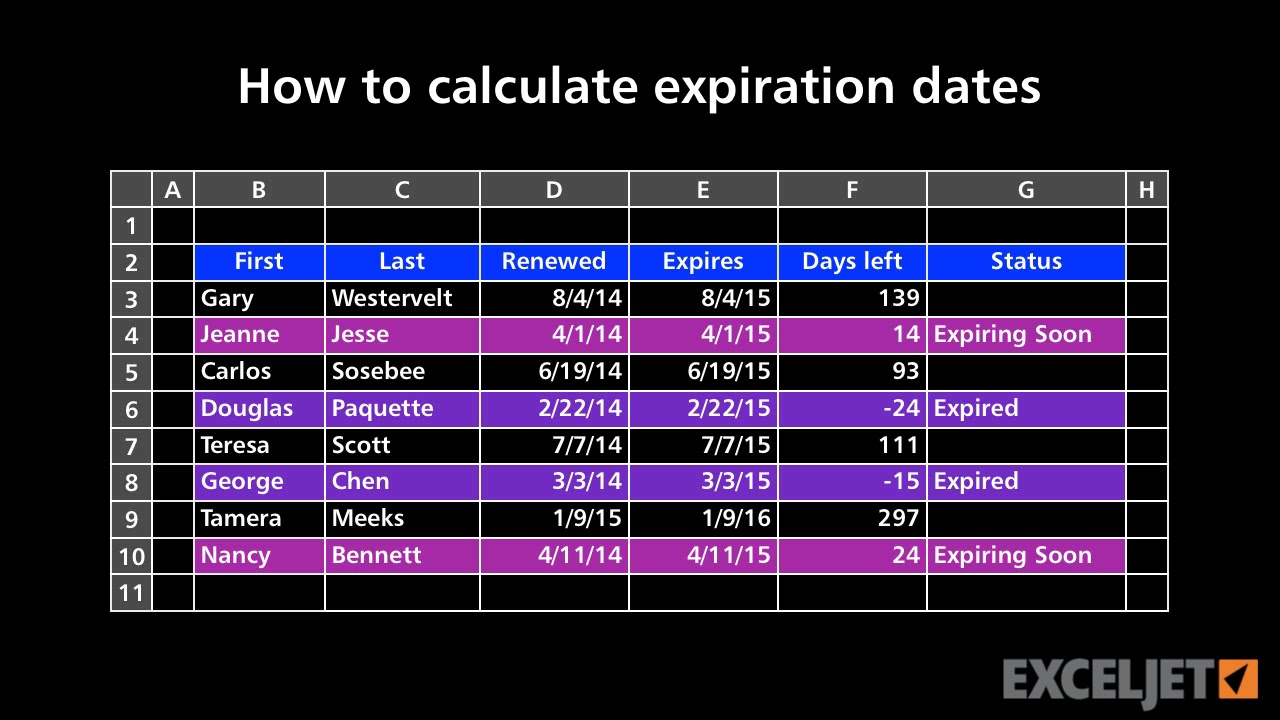
How To Calculate Expiration Dates Youtube

Accelerated Aging Calculator Medical Devices Package Testing Aat Calculator

Loan Amortization Schedule In Excel Amortization Schedule Interest Calculator Excel Tutorials

Shelf Life Calculator For Composites And Other Materials